Dawaiy Medical Company
- Home
- About Us
- Products
- Athomer
- Athomer Jet Isotonic seawater nasal spray (moisturizing & care)
- Athomer Jet seawater nasal spray Propolis
- Athomer Mist seawater nasal spray Mint & Eucalyptus
- Athomer Mist hypertonic seawater spray nasal decongestant
- Athomer Nasal Wash System for healthy nose and sinuses
- Athomer Salt Sachets for nasal wash solution.
- K2Day
- Medic
- Xintamune
- Xintaglobin
- Dermoten
- SunBerg
- Athomer
- Careers
- FAQ
- Contact Us
 العربية
العربية
شركة دوائي الطبية
Medic Ultra Mesh Nebulizer

Chapter 1 Safety Guide
Please read this manual carefully to ensure safe and correct use of this product !
1. Electrical safety
- Classified according to the risk levels of medical devices , Class Ila active ( non-implanted ) medical device
- Classified according to the type of protection against electric shock: Equiment with internal internal power suply;
- Classified according to the degree of protection against electric shock: BF type application part;
- Classified according to electromagnetic compatibility: Group I class B. Shell protection grade: IP22
2. Safety information
✅Warning: Information that you should know in order to avoid injury to patients and medical staff.
✅Note: Important information that should be emphasized.
✅Warning:
🔶Please select the correct drug type, dosage and method of use under the guidance of your doctor.
🔶Do not use any liquid other than your doctor’s prescription to perform the nebulization operation.
🔶Do not use the medicines in suspension or in high viscosity form.
🔶This device is for single patient and shouldn’t be shared with others.
🔶Proper guidance and supervision are required to use this product for children and people with special needs.
🔶This product mustn’t be used in respiratory anesthesia systems and ventilator systems.
🔶This product can’t use oxygen.
🔶Do not store this device in places subject to direct sunlight, high temperature, dusty, lint or water.
🔶Do not carry or store the nebulizer that still has liquid.
🔶Do not immerse the body of this device in water.
🔶Do not disassemble or modify this device.
Note:
🔶The nebulizer can only be used for the specified purpose, i.e. nebulization.
🔶Only use the applicable liquids under the guidance of your physician.
🔶Always clean and disinfect the nebulizer parts in contact with the liquid before using this device.
🔶Remove the stains on the electrodes, or else it may reduce the nebulization effect.
🔶The mask and medicine cup can be disinfected with 75% medical alcohol.
🔶Do not clean the mesh of the medication cup with cotton swabs or brushes.
🔶Before using the nebulizer, you must visually check whether the medication cup is filled with medicine in order to prevent damage to the mesh.
🔶If the instrument isn’t used for a long time,remove the batteries.
🔶Do not drop the nebulizer in order to prevent damage.
🔶Keep it out of reach of infants, children and people with mental illness when storing or using.
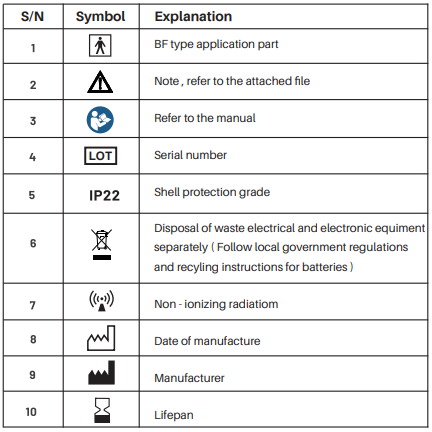
3. Explanation of symbols
4. Environmental protection
The replaced attachments or scrapped product should not be discarded casually. They should be treated according to the environmental protection requirements of the local government to avoid environmental pollution.
Chapter 2 Product Overview
1. Working principle and mechanism of action:
* Working principle:
The working principle of the nebulizer is driven by the rapid oscillation of the circuit, which makes the piezoelectric ceramic transducer oscillate resonantly, thus driving the rapid oscillation of the microporous metal mesh. It converts the electrical energy into mechanical energy and generates ultrasonic vibration, the shock wave squeezes the liquid in the medicine cup, making the liquid pass through tiny mesh on the metal mesh and pop-up quickly, forming countless tiny nebulized particles, which are guided to the patient’s respiratory system through the inhalation mask, so as to achieve the purpose of inhalation treatment.
* Mechanism of action:
The respiratory system is an open system, after the liquid is nebulized into particles and inhaled by the patient, the drug mist can directly adsorb on the patient’s mouth, throat, trachea, bronchi and alveoli, and is absorbed by the mucous membrane to achieve the purpose of treatment.
2. Product composition:
It mainly consists of a main unit, a medication cup,two inhalation masks
3. Applicable people:
It’s suitable for breathing administration of adults and elderly, infants and children should be used under adult supervision.
4. Application scope:
Nebulized medication for therapeutic use.
5. Intended environment:
It’s suitable for medical institutions (such as hospitals, clinics, health centers, etc.) and home nebulization.
6. Contraindications:
Patients with acute cardiopulmonary failure or airway obstruction are prohibited. Do not use the nebulizer if you are allergic to aerosolized drugs.
7. Product performance:
Power supply : 2×1.5V AA alkaline batteries, or 5 V 1A(adaptor with IEC60601- 1 approval) Power: < 3.0 W
Ultrasonic oscillation frequency : Nominal frequency of the nebulizer is 110 KHz, and the deviation of the ultrasonic oscillation frequency of the nebulizer from the nominal frequency is ≤ ±10 %
Maximum nebulization rate: ≥ 0.2 ml / min
Temperature inside the sink : ≤ 60 ˚C
Operating noise: ≤ 50 dB ( A weighting )
Low water level prompt or shutdown: the instrument will stop automatically after the liquid atomization is completed
Continuous working time (battery life): In the fully charged state, the nebulizer could be able to work continuously for 30 minutes.
Median particle size of the mist particles: The median size of the nebulizer mist particles is 3 μm, and the error should not exceed ±25%;
The proportion of the mist particles with a diameter of 1um to 5um should be greater than 50%.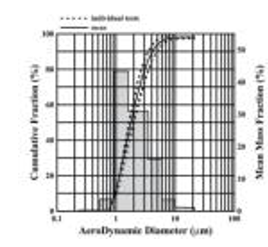
Distribution of equivalent volume and particle size of fog particles Test conditions: temperature range: 23 ± 2 C, humidity range: 56 % ~ 59 %; The composition of the test solution is 0.9 % sodium chloride solution.
(test data may vary with test conditions and drug solution)
Nebulization rate: Low, medium and high
Maximum capacity of medication cup: 8 ml
Product size: 48 ( L ) X 64 ( W )X 126 ( H ) mm
Weight: About 98.6 g ( excluding battery )
Operating environment
Temperature: 5 ˚C~40 ˚C
Relative humidity:
≤ 80 % , non-condensing
Atmospheric pressure: 86.0~106.0 kPa
Transportation/storge environment
Temperature: -20˚C ~ 55˚C
Relative humidity:
≤ 93 % , non-condensing state
Atmospheric pressure: 70.0~106.0 kPa
8. Configuration list:
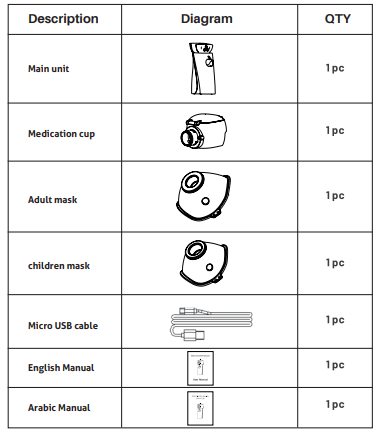
Note: the above parts are necessary for normal use of the product and comply with relevant standards.
9. Key components list:
The key component of this product is an ultrasonic transducer.
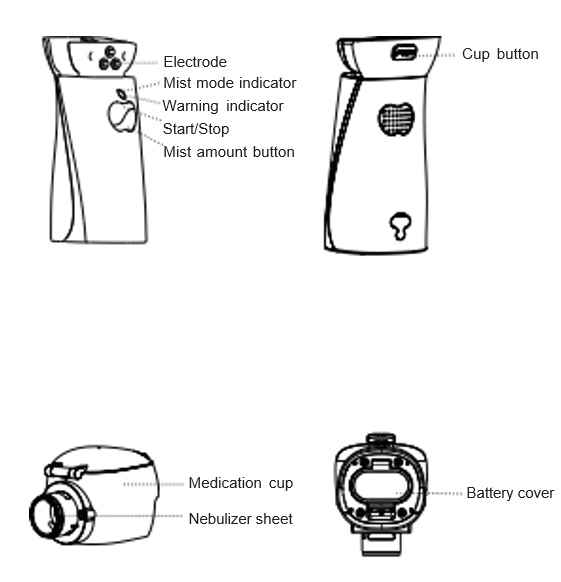
Chapter 3 Product Appearance
Chapter 4 Power Use
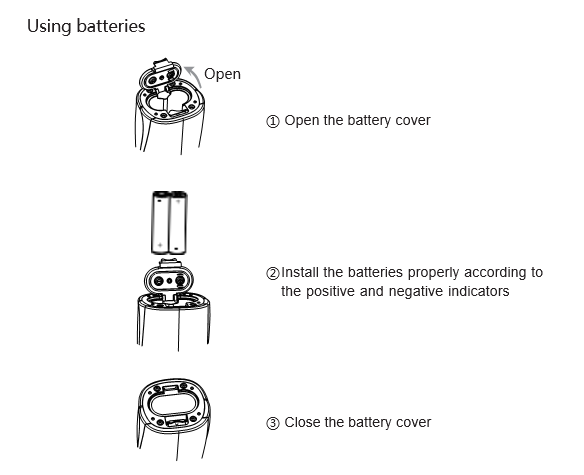
Note:
1 . When the battery is low and the mist mode indicator flashes, please replace the batteries as soon as possible.
2 . Do not short circuit the batteries or reverse the positive and negative poles, or else it may cause serious damage to the equipment.
3 . If the instrument isn’t used for a long time, remove the batteries from the battery compartment.
4 . The batteries must be disposed of in accordance with local regulations after use.
Chapter 5 How to Use?
The medication cup and mask should be cleaned and disinfected before use Each component needs to be cleaned, disinfected and dried before installation.
1. Install the medication cup on the main unit:
( When installed properly, you will hear a click sound, indicating that the installation is in place )
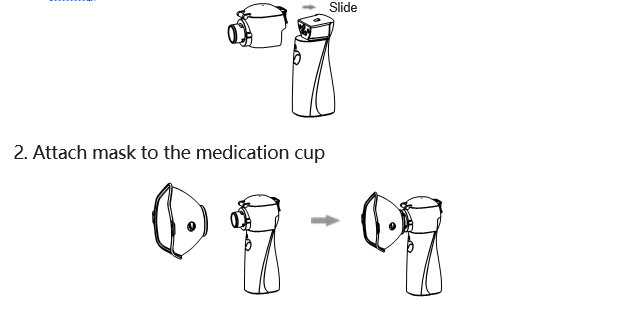
Note: Please select the correct mask.
a) Large mask are suitable for adults, small mask is suitable for children.
b) The mask can be reused 20 times; If necessary, please contact our company or purchase it with regular channels such as drug stores and hospitals.
c) Please operate in reverse order to remove.
3. Pour the liquid into the cup and fasten the lid to prevent the liquid from leaking out:
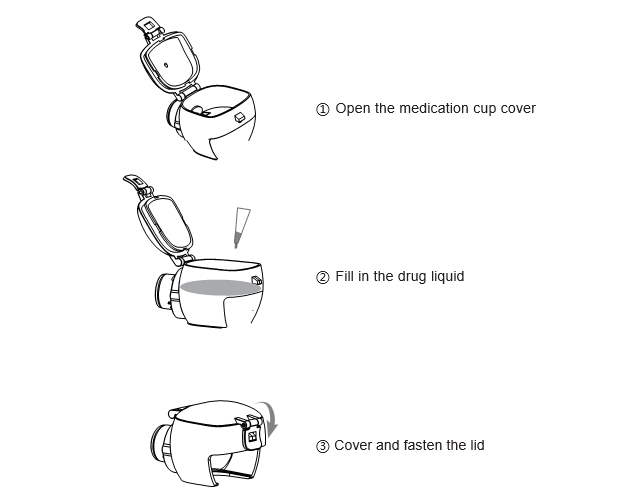
Note: Please select the correct mask.
Please consult your physician for the dosage of the drug liquid. Maximum capacity 8ml, Minimum capacity 0.5 ml.
Do not use suspended, highly viscous or high-concentration liquids, as this may cause the nebulization sheet clogged or damaged and not function properly.
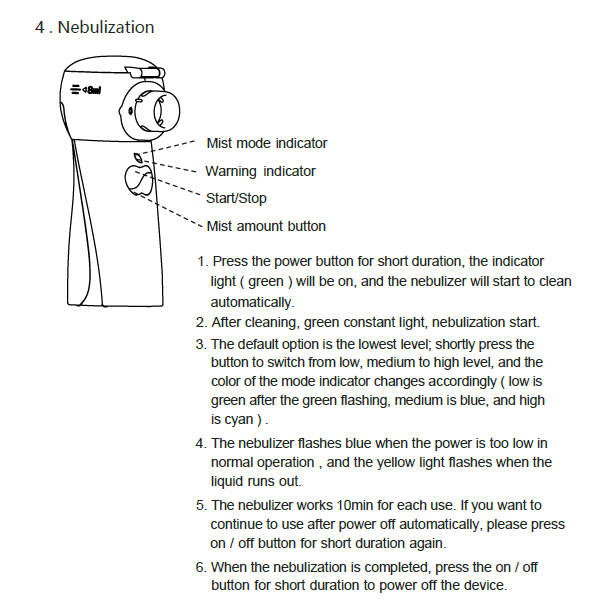
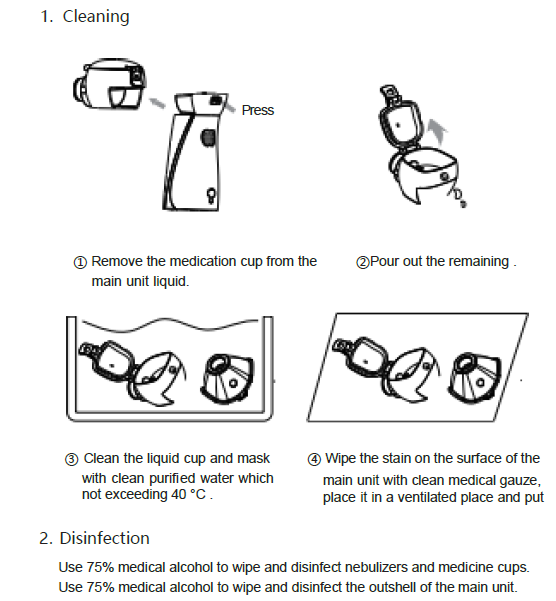
Chapter 6 Cleaning and Disinfection
After nebulization, pour out the remaining liquid, clean and disinfect the nebulization device and the medication cup .
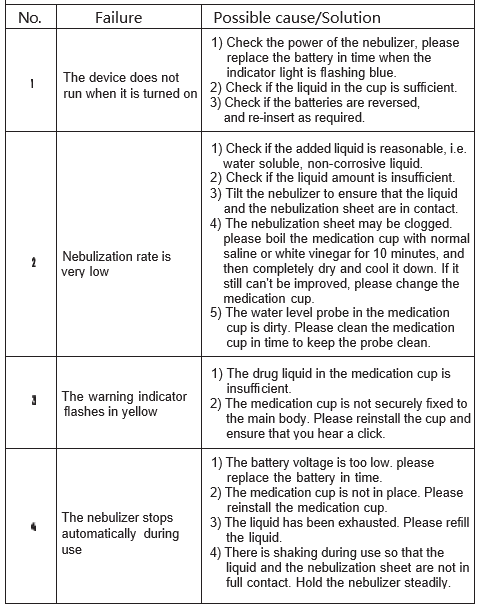
Chapter 7 Troubleshooting
If you have any problem or doubt when using the nebulizer, please troubleshoot according to the following list.
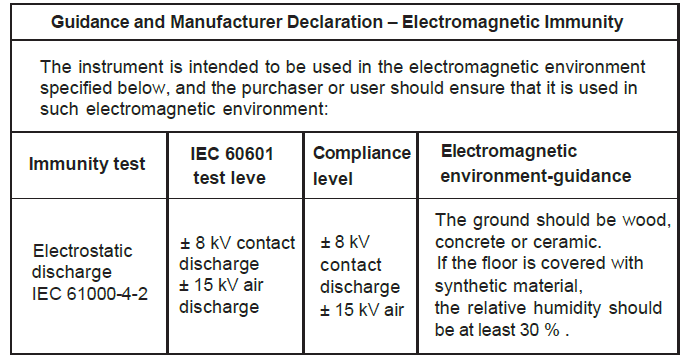
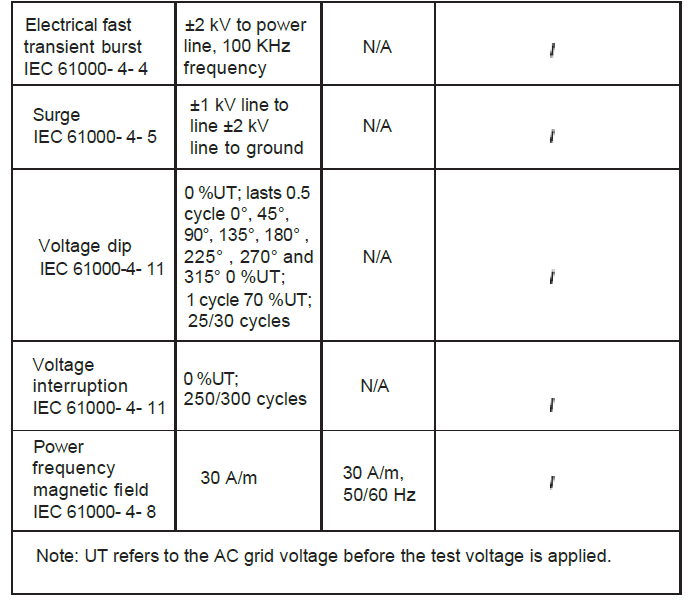
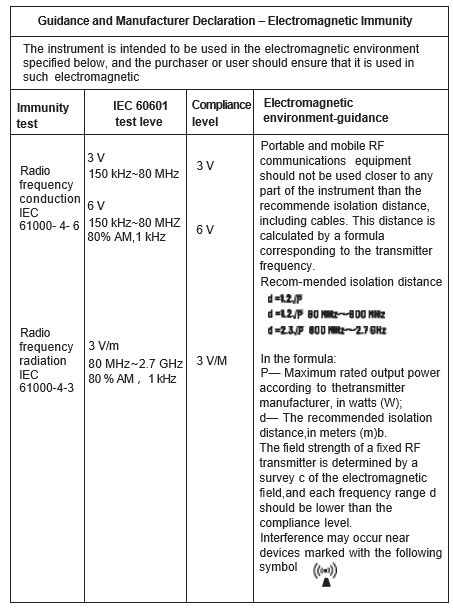
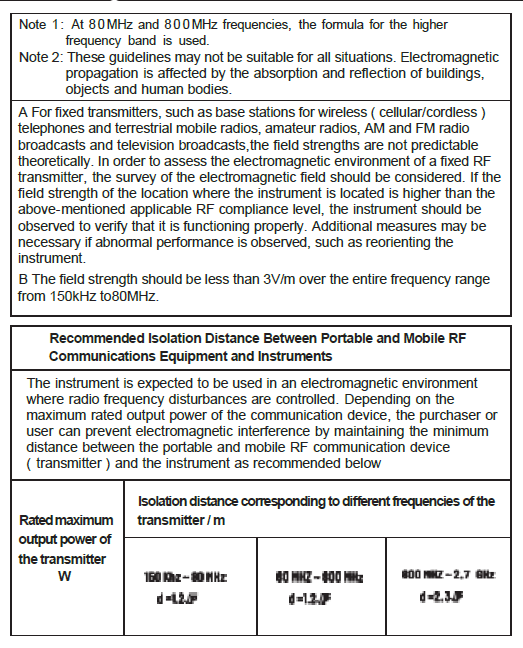
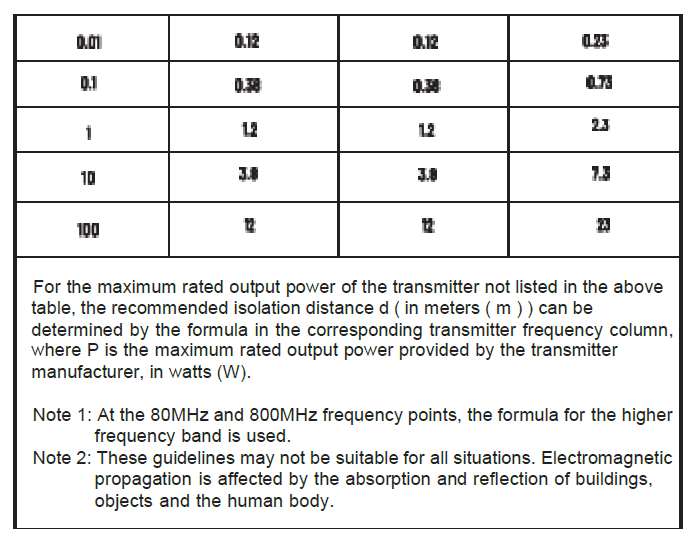
Chapter 8 EMC
Note:
- The instrument conforms to the requirements of IEC60601- 1 – 2 standard for electromagnetic compatibility.
- The user shall install and use the EMC information provided in the random file.
- Portable and mobile RF communication equipment may affect the performance of the instrument, avoid strong electromagnetic interference when using, such as close to the mobile phone, microwave oven, etc.
- The guidance and manufacturer’s declaration are detailed in the table below.
Warnings:
- The instrument should not be close to or stacked with other equipment.
If it must be close to or stacked, it should be observed and verified to be able to operate normally under its configuration.
EMC

Chapter 9 Warranty
- If the product has a fault, please contact your local dealer or manufacturer.
- This product from the date of sale: the main unit is free warranty for 1 year, medicine cup (including nebulizer sheet) warranty for 3 months.
- We will charge for relevant fees as appropriate when the warranty period expires.
- Consumables (masks) are not covered by the warranty.
- The following conditions are not covered by the free warranty:
* Damage caused by drop, impact, soaking in water or getting wet.
* Damage caused by improper operation.
* Damaged caused by an accident.
* Failure caused by reconstructing or altering the altering the original structure.
Disclaimer: We are not responsible for any problem of the instrument if any parts are repaired by unqualified personnel.
Use period:Three years.
Software version:V1.0.0. 1
Version: V1. 1
Created Date :2019.08
Date of revision: 2019.12
